RESEARCH IN APPLIED MICROBIOENGINEERING
Noncanonical Wnt Activation Promotes the Mechanotransduction of Cytotoxic CD8+ T-Cells
Mechanical Force Regulation of Asymmetric Vascular Cell Alignment

MICRO-MECHANO BIOENGINEERING INTRODUCTORY VIDEO
I spent the summers of 2020 and 2021 conducting research for New York University’s Applied Micro-Bioengineering Laboratory. My team and I collaborated on this video to introduce our field of research and the laboratory’s motivations.
In this video, we discuss how micro-mechano bioengineering is a multidisciplinary field that intersects the usage of mechanics, electronics, and biological processes. Its applications allow for disease modeling though controlled environmental advancements. Our research specifically sought to bridge the fields of mechanobiology, tissue engineering, regenerative medicine, and cancer biology.
From 1:30 to 2:05, I discuss micro-fabrication protocols and their pertinence to our research objectives.
ABSTRACT #1
-
Noncanonical Wnt Activation Promotes the Mechanotransduction of Cytotoxic CD8+ T-Cells
This project utilized force analyzation devices and algorithms for the tracking and subsequent computational analysis of force generation during the activation of CD8+ cytotoxic T-Cells. Stronger immune responses exhibited positive correlation with higher levels of total cellular forces during CD8+ T-Cell activation. The potential signaling pathways to augment the mechanotransduction and immune responses remain largely unexplored. Here, we explored the effects of noncanonical Wnt activation on T-Cell mechano-transduction to find an effective molecular pathway to elevate the cytotoxicity of CD8+ T-Cells and increase the efficiency of immunotherapy by conjugating the designated activating antibodies and noncanonical WNT- activating ligand, Foxy5 peptides, on the PDMS micropillar force measure device. Results showed that Foxy5-mediated noncanonical Wnt activation effectively increased the force feedback in activated CD8+ T-Cells. This research furthers the field of mechanoimmunology by providing a novel signaling pathway to modulate the CD8+ T-Cell activation.


ILLUSTRATED METHODOLOGY

SUMMARY OF FINDINGS
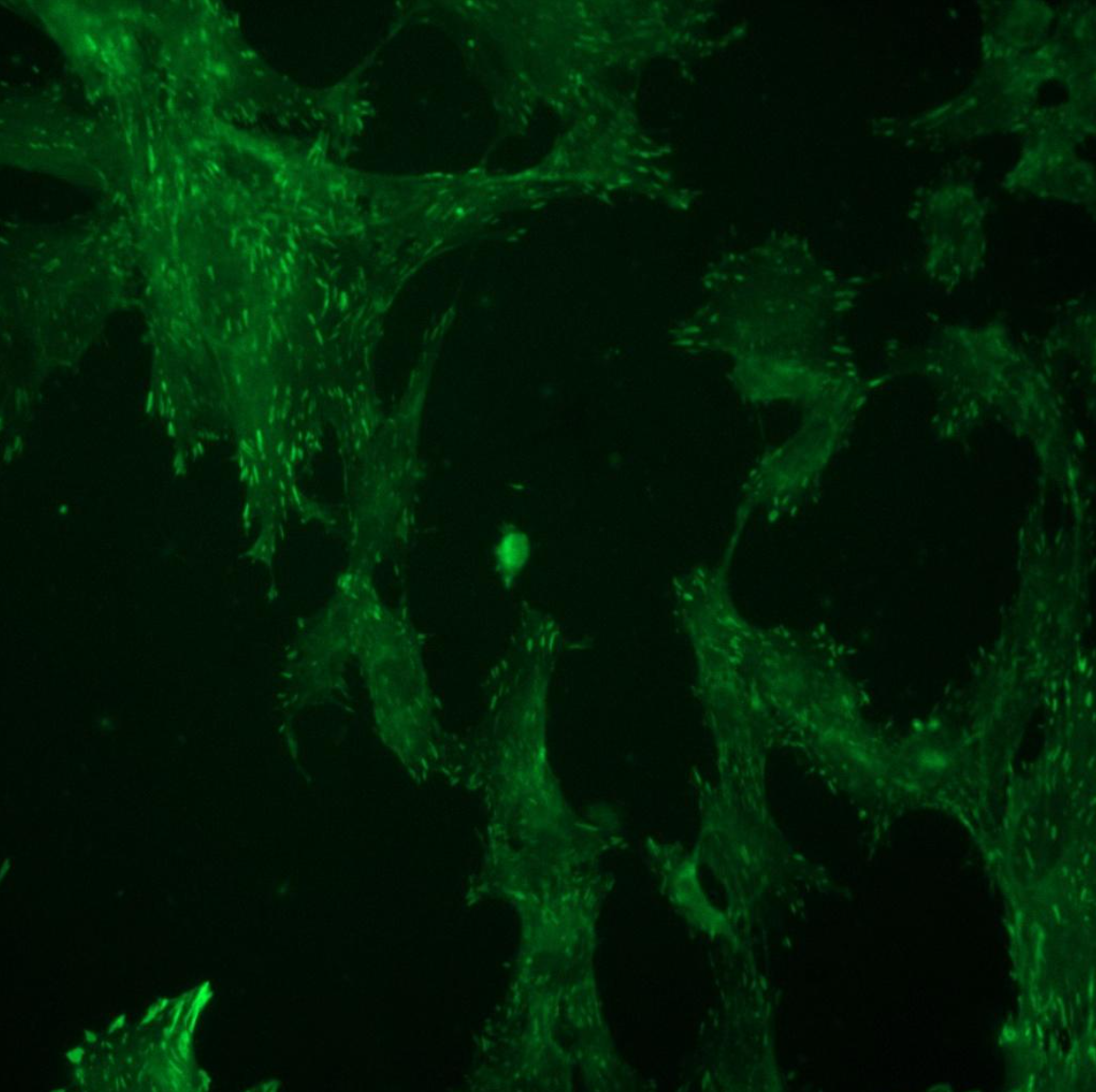
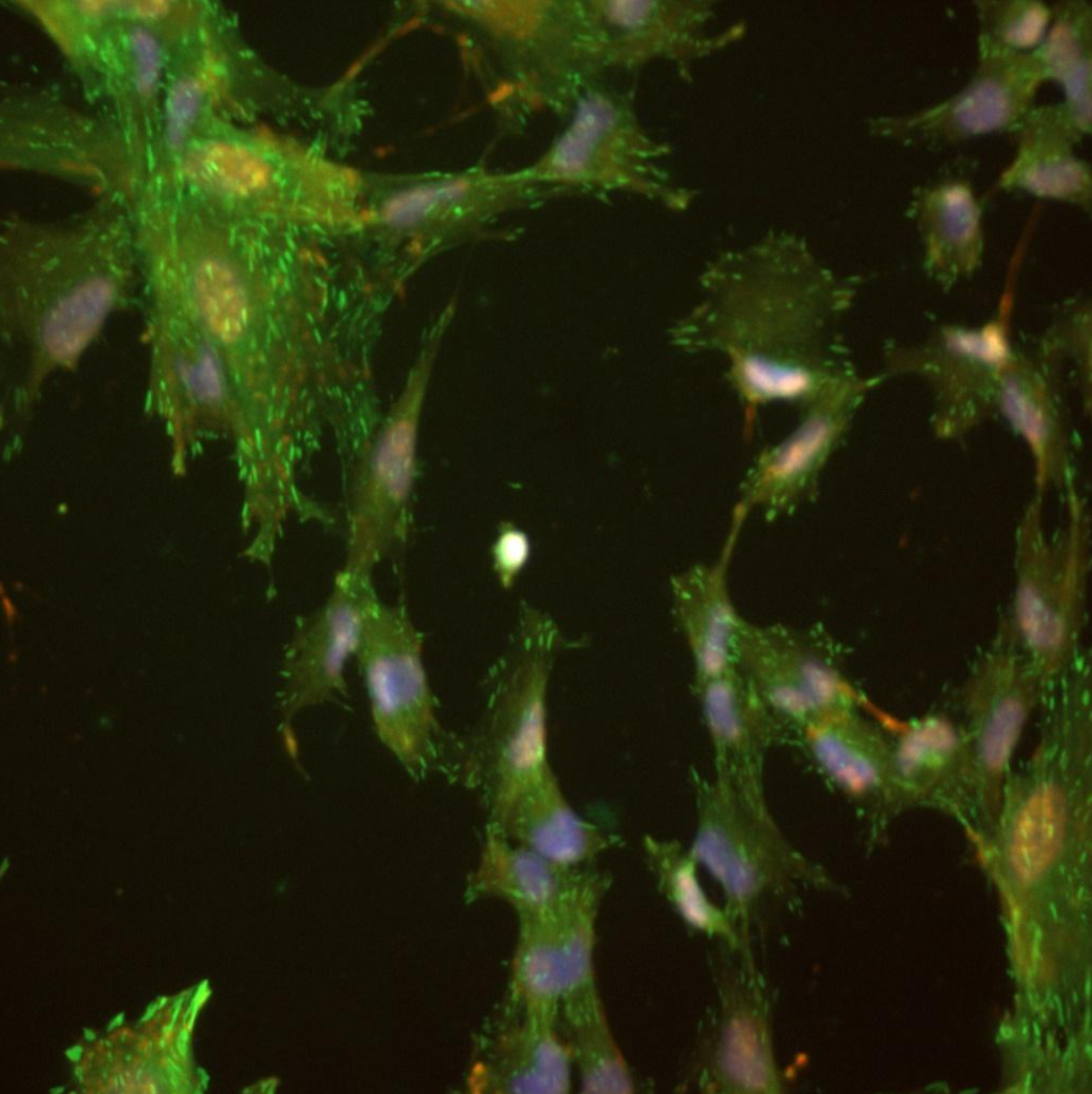
Micro-pillar displacement metrics and fluorescent imaging were both critical to our investigation into how foxy5 peptides modulate cytotoxic CD8+ T-cell activation and mechanotransduction. The metrics quantified the direction and magnitude of forces involved, while the staining and imaging techniques provided a visualization of the cells after activation. Together, these methods offered a comprehensive approach to understanding the underlying mechanisms of T-cell activation and the cytotoxic immune response. By elucidating these mechanisms at the cellular level, this research can contribute to the development of novel strategies for enhancing immune function against infection and disease.
Force direction dynamics data shows clear spreading movement (positive) after Foxy5 activation, and minimal spreading movement after Src (control) activation. These contrast to squeezing movement (negative).
Force dynamics data shows clear differences in the strengths of forces produced by T-Cells when activated by Foxy5 versus Scr (control).

My team’s research on this project was published to The Royal Society of Chemistry’s Chemical Communications Journal: Issue 94, 2021.
DOI: 10.1039/D1CC05194F
ABSTRACT #2
-
Mechanical Force Regulation of Asymmetric Vascular Cell Alignment
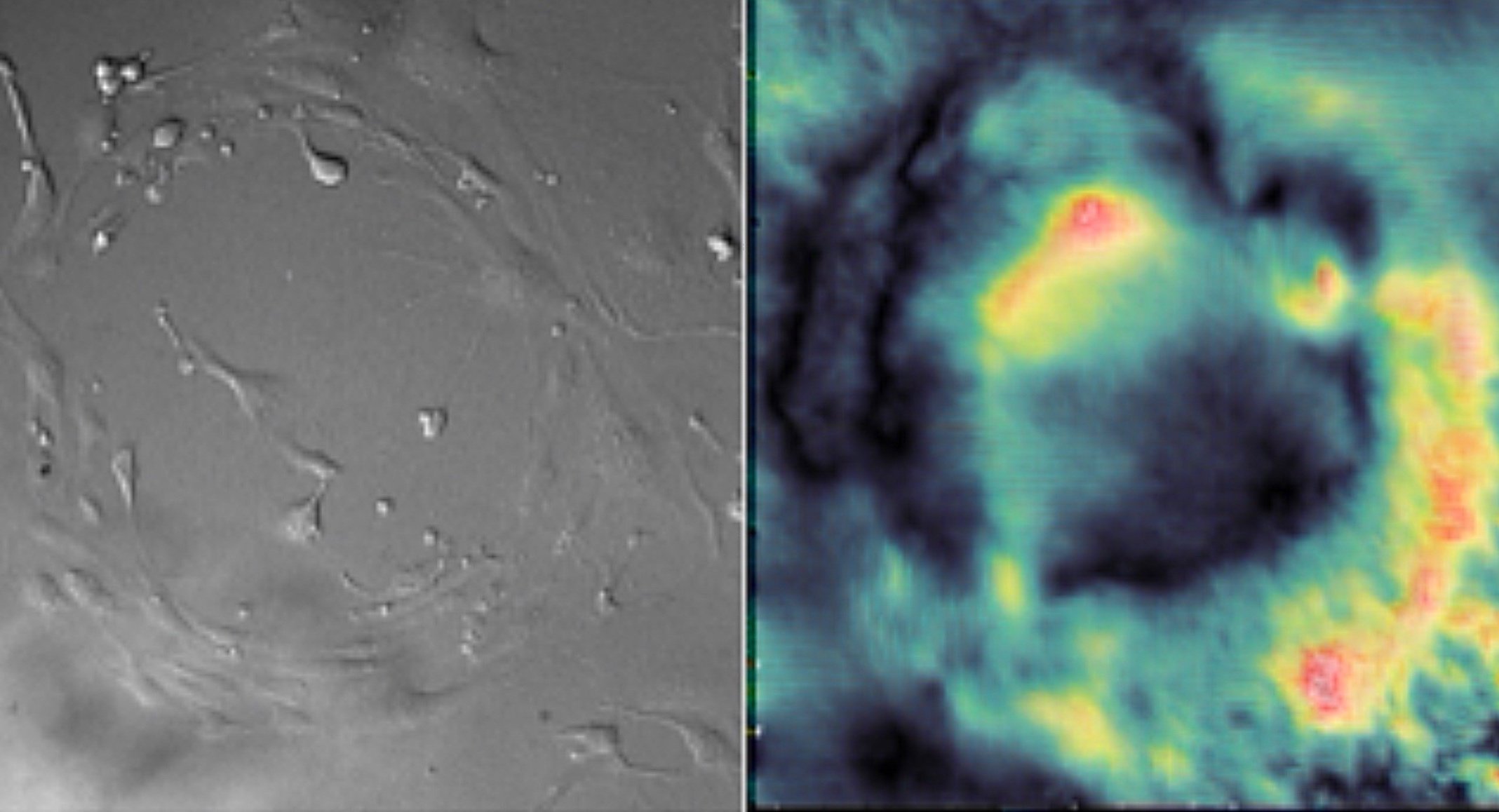
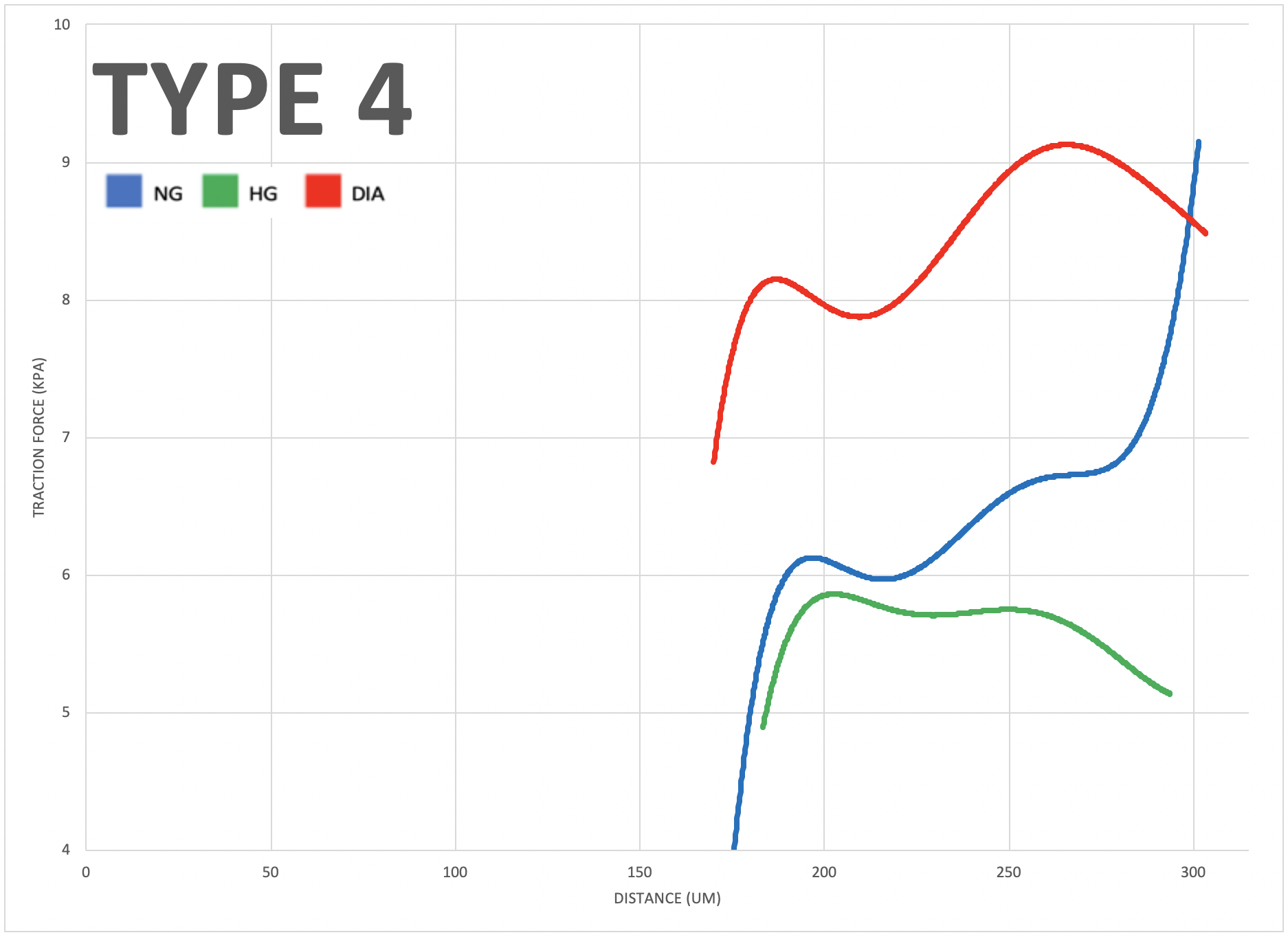
This study investigates the role of mechanobiology in vascular endothelial cells by comparing the mechanical characteristics and multicellular coordination abilities of healthy and diabetic aortic-endothelial cells (ECs). Cells experience mechanical forces and external stressors in their microenvironment that require appropriate cellular responses to maintain physiological functioning. The cultures were grown on mechanical force detecting substrates with pharmacological treatments and varying glucose concentrations to simulate and regulate morphogenesis in vitro.
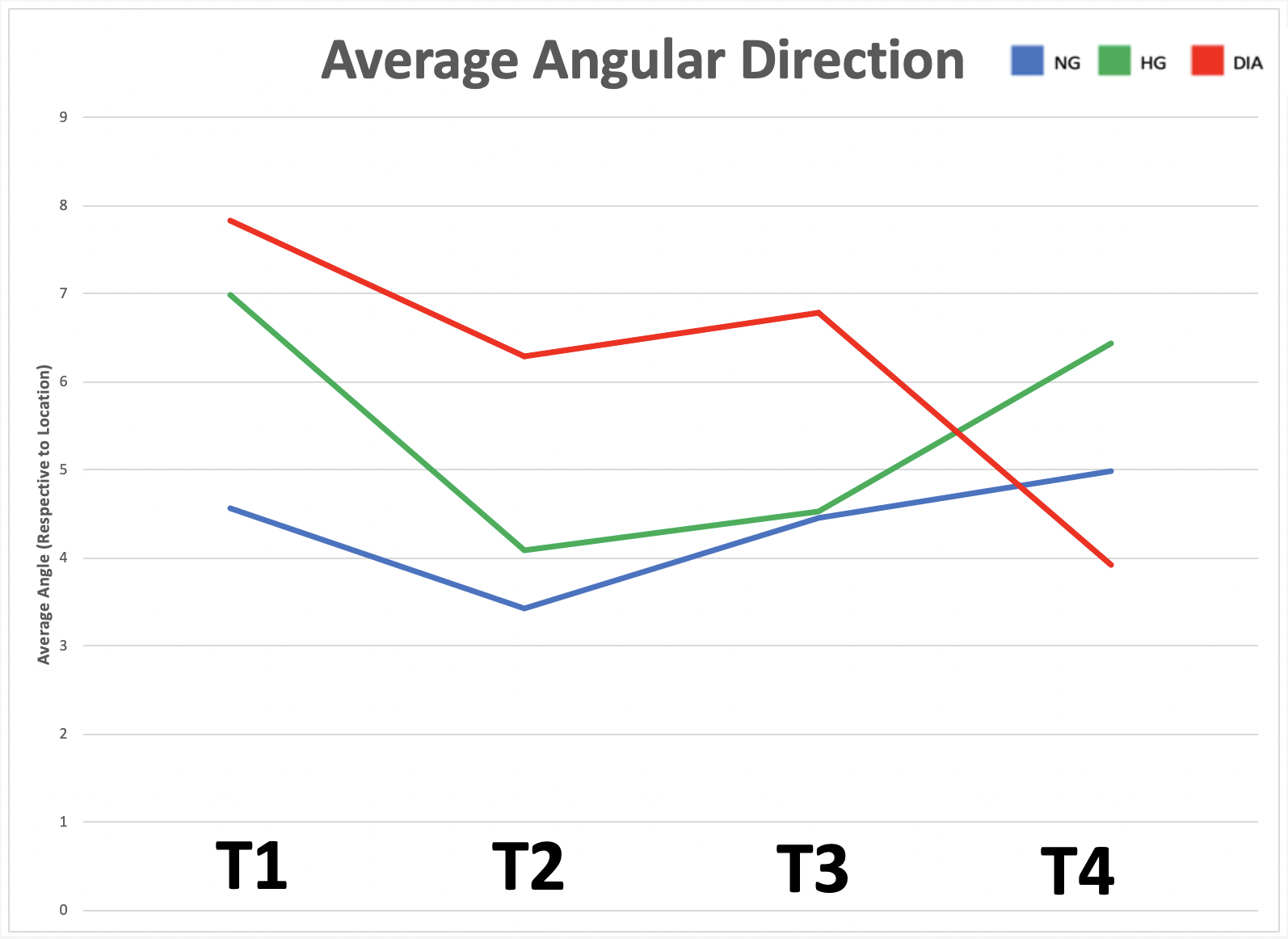
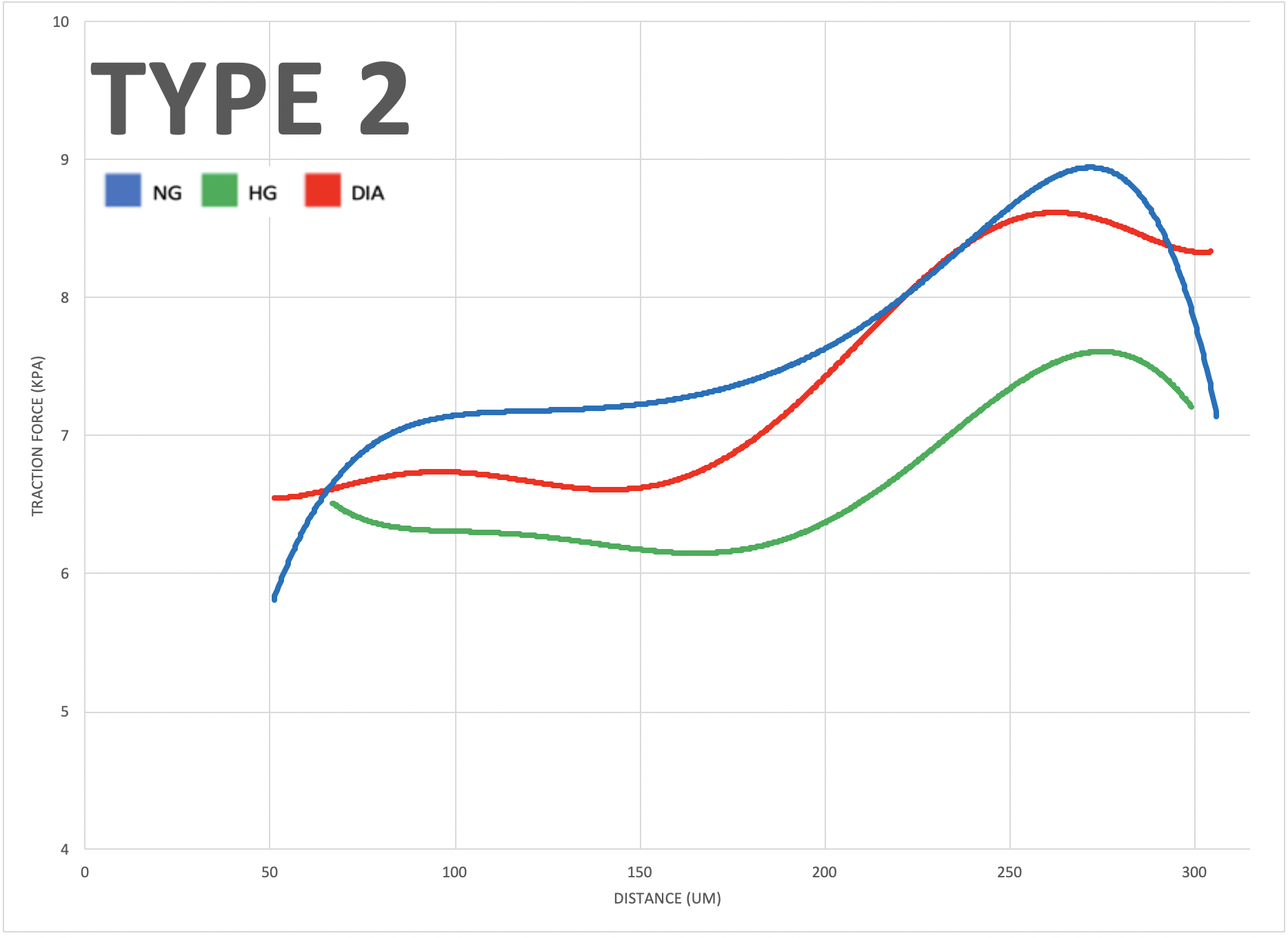
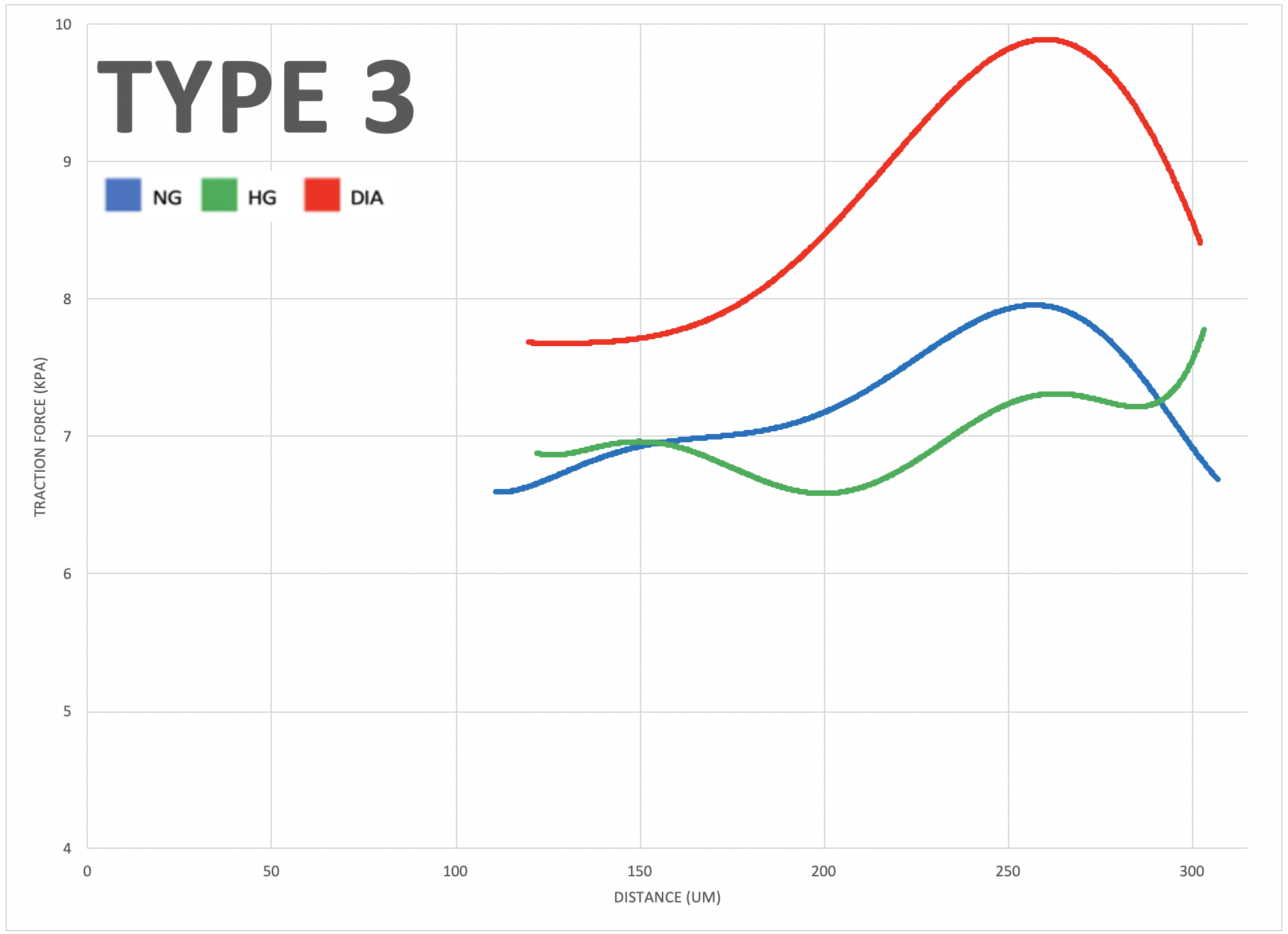
To investigate the impact of varying glucose conditions and simulated disease states, traction force microscopy (TFM) was employed to visualize the cell alignments and forces across different tissue geometries. The resulting data showed that healthy and diseased ECs exhibit unique mechanical properties and coordination abilities, as indicated by particle displacement in the substrate. These findings suggest that certain patterns of asymmetry may be related to cardiovascular diseases, highlighting the importance of mechanobiology in the adaptation of vascular systems. Overall, this study sheds light on the role of mechanical forces and external stressors in cellular responses and their impact on physiological functioning, providing valuable insights into the interplay between mechanical forces, cellular responses, and vascular physiology that could have important implications for the development of treatments for cardiovascular diseases.


SUMMARY OF FINDINGS
The study aimed to investigate the role of mechanobiology in disease states of vascular endothelial cells. The results showed that diabetic cells experienced greater traction forces than healthy cells when greater mechanical stress was applied, indicating specific force patterns are exhibited by diseased cells. The study also revealed that high glucose levels impact multicellular cooperation, reflected through their average orientation. Additionally, negative control, positive control, and diabetic cells, respectively, showed increasing degrees of either clockwise or counterclockwise orientation in a multicellular system, indicating an impact of disease on coordination abilities. Overall, this study offers valuable insights into the interplay between mechanical forces, cellular responses, and vascular physiology, which could have significant implications for early diagnostics of cardiovascular diseases.
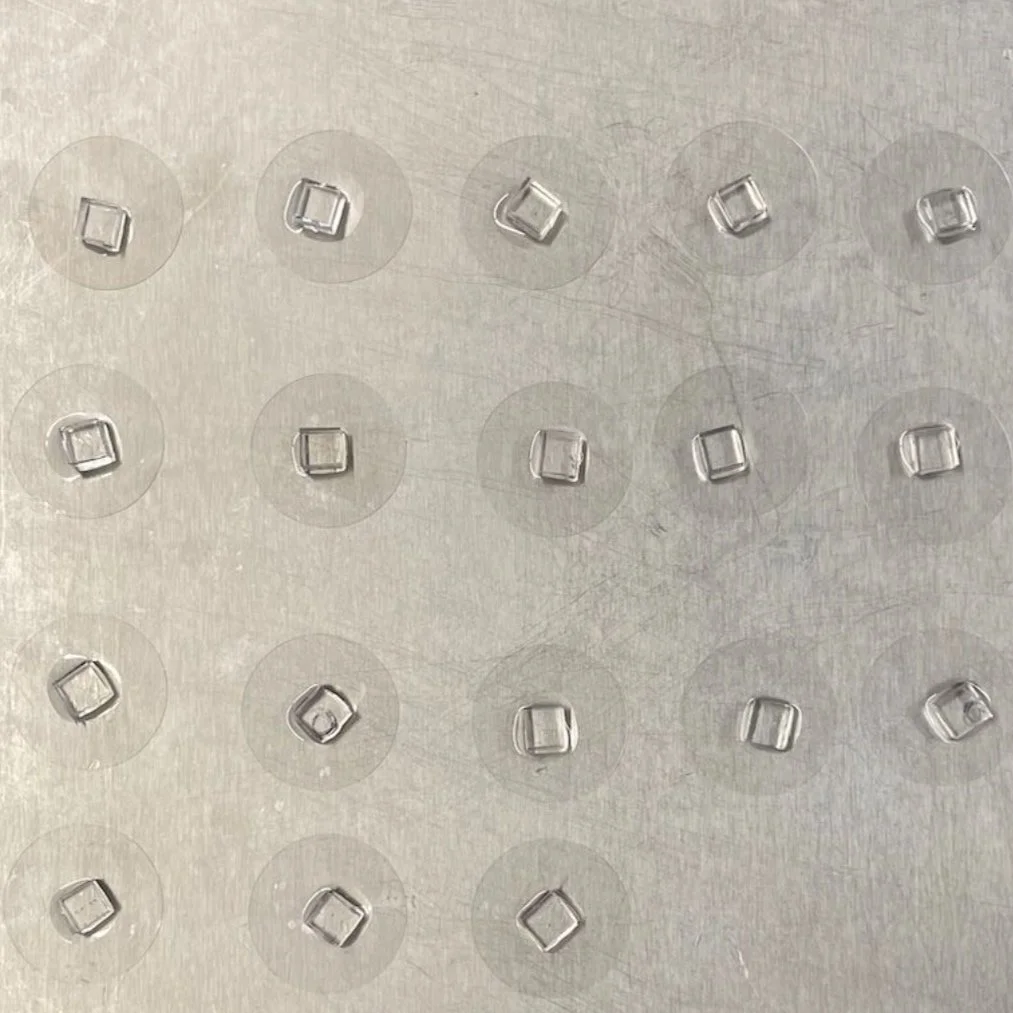
Micro-contact printing (fibronectin stamping) allows cells to grow in specified areas of the petri dish, producing different tissue geometries. The four types shown here (Type 1 to 4) result from fibronectin stamping and simulate varying levels of mechanical stress placed on vascular endothelial cells in the human body (increasing from left to right)."